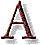
s most of our regular customers know,
Alpha Omega Labs kept some administrative functions
in Nassau, Bahamas; and the balance of its operation
in Lake Charles, Louisiana, up until it was raided
by U.S. Government authorities on September 17, 2003.

AO shipped product
both to end users and
distributors
around the world. During 2002-2003, AO used RisingSun
Health, a company in Montana, run by an aspiring
politico named Toby McAdams, to handle Alpha Omega
shipments within the U.S.

The relationship
soured in the summer of 2003 because of payables
in arrears with McAdams' operation that topped
$9,000 (of which $6,000 is still owed to AO).

On the surface
of things, this is mindless gossip and shouldn't
pertain to any of our customers. What
DOES
pertain to our customers, however, is the illegal
use of our trademark and the deliberate
adulteration and mislabelling of the product.

Mr. McAdams
has been bragging that the FDA has assisted him
in disposing of AO in the U.S. and "approves" of
his Cansema. We have serious doubts as to the
truthfulness of this statement -- however,
if true,
it represents a level of deceitfulness in dealing
with the public that exceeds anything even WE have
seen on the part of this unscrupulous agency.

We received the above product
from an unhappy customer in Tennessee who wanted us to evaluate it.
The following evaluation is organoleptic (there was no time to
do analytical chemical analysis) by two of our most experienced
herbalists, including Greg Caton himself. Here is their (and our)
joint statement:
 "By any standard that the FDA has ever set,
RisingSun Health's product is both "mislabelled" and "misbranded" --
indeed, to such an extreme degree that is cannot be anything
but deliberate. Small items may have been perhaps an oversight:
the lack of a net weight, the use of the word "dosage" instead of
"instruction" for something that is not supposed to be an
"unapproved drug." These are triffling matters -- again,
perhaps an oversight.
"By any standard that the FDA has ever set,
RisingSun Health's product is both "mislabelled" and "misbranded" --
indeed, to such an extreme degree that is cannot be anything
but deliberate. Small items may have been perhaps an oversight:
the lack of a net weight, the use of the word "dosage" instead of
"instruction" for something that is not supposed to be an
"unapproved drug." These are triffling matters -- again,
perhaps an oversight.
 "What is far more serious
is the contents of the product is not remotely the same as the
"ingredient declaration" on the product itself. The declaration
is an exact duplication of Alpha Omega's -- along with nearly
all the instructions and related support documentation on the
risingsunhealth.com web site. See our Cansema FAQ, question 200.
"What is far more serious
is the contents of the product is not remotely the same as the
"ingredient declaration" on the product itself. The declaration
is an exact duplication of Alpha Omega's -- along with nearly
all the instructions and related support documentation on the
risingsunhealth.com web site. See our Cansema FAQ, question 200.
 "He just copied and pasted our declaration.
"He just copied and pasted our declaration.
 "Even that isn't an issue.
"Even that isn't an issue.