|
Traditional herbal for hair loss, respiratory
disorders, and regulation of menstruation
Avenca

Avenca
(Adiantum capillus-veneris)
Code BOS212
Price: $18.95
120 Capsules x 500 mg.
Order Now
 Email
Email
 Summarized Description: Summarized Description:
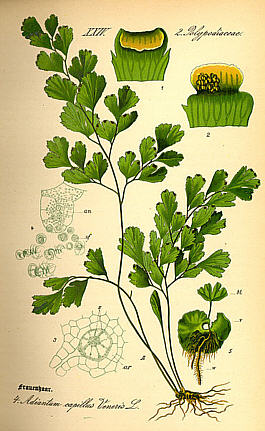
Known as "Maidenhair Fern" in the U.S., Avenca has broad geographic distribution throughout
the world's rainforests, from the near tropical temporate zones well into the equatorial zone.
(We obtain our variety from the Peruvian Amazon.)
With leaves up to 50 cm. long, it can reach a height of 30 cm. in height,
making it a common houseplant and garden fern. Ethnobotanically, this herb is
used to treat a broad range of ailments, including rheumatism, non-specific
mental disorders, respiratory ailments, hair loss, contraception, and to regulate menstruation.
 Besides Maidenhair Ferm, other common names for Avenca
include Venus Hair Fern, Adianto, Cabello de Venus, Culantrillo, Doddergrass, Pursha, and Shopumbillo,
among many others.
Uses & Protocols
 Avenca is normally taken as a leaf infusion or root
tincture. We provide the herb in the 120 capsule format. Initial Dosage: One capsule, 2x a day, unless
otherwise directed by your naturopathic physician.
Warnings & Contraindications
 Those with hypoglycemia or diabetes should taken
with caution, monitoring their blood sugar levels accordingly. Avenca should not be taken
by pregnant women or women looking to conceive or women with estrogen-positive cancers.
According to Taylor, Avenca "may potentiate insulin and antidiabetic drugs."
Shelf-Life
 Five years or more.

Medicinal Activities
 Further information for practitioners: Further information for practitioners:
World-famous botanist Dr. James Duke attributes the following activities
to this plant (p. 18-20; see hardcopy cover at right,
purchasable on Amazon),
drawn from the extant literature. (See his graduation for "level of
efficacy" on our amazon traditionals page;
followed by Duke's bibliographic abbreviations (in capital letters),
which we identify
on a separate page.)
 Duke provides a " food farmacy potential" score for this
plant of "FNFF=!."
- Abortifacient (F; EB47:184)
- Analgesic (f; NPM)
- Anthelmintic (f; LMP)
- Antibilious (f; DEP)
- Antifertility (f1; RAI)
- Antioxidant (f; RAI)
- Antiradicular (f; RAI)
- Antiseptic (f1; RAI)
- Antitussive (f; EGG; RAI)
- Antiviral (1; RAI)
- Astringent (1; BUR; HHB)
- Bactericide (f1; RAI)
- Cardioprotective (f; RAI)
- Contraceptive (f1; EB31:340; RAI)
- Decongestant (f; RAI)
- Demulcent (f; GMH; PH2; VAD)
- Deobstruent (f; BOU; DEP)
- Depurative (f; DAA; EB47:184)
- Detoxicant (f; RAI)
- Diaphoretic (f; DAA; EGG)
- Discutient (f; DEP)
- Diuretic (f; BOW; DAA; DEP; NAD)
- Emetic (f; DAA; LMP)
- Emmenagogue (f; BUR; DEP; HHB; KAB; NAD)
- Emollient (f; BOU; DAA; EGG; UPW)
- Expectorant (f; BOW; BUR; DAA; DEP; NAD; PH2; UPW)
- Febrifuge (f; DAA; DEP; LMP)
- Hemostat (f; EB31:340)
- Hepatoprotective (f; RAI)
- Hypocholesterolemic (f1 RAI)
- Hypoglycemic (1; HH2; RAI)
- Hypotensive (f1; RAI)
- Laxative (f; DAA)
- Mucolytic (f; VAD)
- Pectoral (f; BUR; DAA; DEP; GMH; PH2; UPW)
- Propecic (f; DAA; GMH; PH2)
- Refrigerant (f; BUR)
- Resolvent (f; DEP)
- Secretolytic (f; RAI)
- Stimulant (f; DAA; EFS; GMH)
- Sudorific (f; DAA)
- Tonic (f; BUR; DAA; UPW)
Indications
 Further information for practitioners: Further information for practitioners:
Duke provides the following indications for this plant:
- Alopecia (f; BOW; DAA; RAI)
- Amenorrhea (f; AUS; KAB; EB50:40)
- Asthma (f; AUS; DAA; GMH)
- Bacillus (1; RAI)
- Bacteria (f1; RAI)
- Biliousness (f; DEP)
- Bleeding (f; MAX)
- Boils(f; NPM)
- Bronchosis (f; HH2; KAB; PH2; EB48:146)
- Cancer (f; DEP)
- Cancer, liver (f; UPW)
- Cancer, spleen (f; UPW)
- Cancer, uterus (f; UPW)
- Candida (f1; NWO; RAI)
- Cardiopathy (f; RAI)
- Cancer, brain (1; RAI)
- Catarrh (f; DAA; DEP; GMH)
- Cephalosis (f; DAA)
- Cerebrosis (f; AUS)
- Chest Colds (f; UPW)
- Childbirth (f; DAA; EB50:40; JFM)
- Chills (f; DAA; EB28:327)
- Colds (f: DAA; EB48:146; KAB; MKK; UPW; WO3)
- Colic (f; KAB)
- Congestion (f; RAI)
- Constipation (f; DAA)
- Consumption (f; AUS)
- Coughs (f; AUS; EB48:146; EGG; GMH; HH2; NAD; NPM; PH2; RAI)
- Cystosis (f; DAA)
- Dandruff (f; BOW; NWO)
- Diabetes (f1; HH2; RAI)
- Dropsy (f; DAA)
- Dysmenorrhea (f; AUS; DAA; HH2; NPM; PH2)
- Dysuria (f; EGG; RAI)
- Eczema (f; RAI)
- Escherichia (1; RAI)
- Fever (f; BUR; DAA; DEP; LMP)
- Flu (f; RAI)
- Fungus (f; NWO)
- Gallstones (f; RAI)
- Gastrosis (f; RAI)
- Gigivosis (f; VAD)
- Gout (f; GMH)
- Gravel (f; AUS; DAA; GMH)
- Gray Hair (f; PH2)
- Headache (f; NPM; WO3)
- Head Colds (f; DAA; KAB; UPW)
- Heartburn (f; RAI)
- Hepatosis (f; BOU; DAA; JFM; RAI; UPW)
- High Blood Pressure (f1; RAI)
- High Cholesterol (f1; RAI)
- Hydrophobia (f1; RAI)
- Hyperglycemia (1; HH2; RAI)
- Impetigo (f; LMP)
- Infection (f1; BUR; HHB; NWO; RAI)
- Infertility (f; NWO)
- Insanity (f; DEM)
- Jaundice (f; AUS; EGG; GMH; RAI)
- Kidney Stones (f; AUS; RAI)
- Laryngosis (f; RAI)
- Lice (f; NWO)
- Metrorrhagia (f; EB31:340)
- Mucososis (f; RAI)
- Mycosis (f; NWO)
- Nephrosis (f; AUS; GMH)
- Pain (f; NPM; PH2)
- Periodontosis (f; VAD)
- Pertussis (f; HH2; PH2)
- Pharyngosis (f; BOW; RAI; VAD)
- Placenta (f; EB50:40)
- Pleurisy (f; AUS; GMH; RAI)
- Pseudomonas (1; RAI)
- Pulmonosis (f; DAA; DEP; EFS; GMH; KAB)
- Respirosis (f; DAA; HHB; PH2)
- Rheumatism (f; DEM)
- Rhinosis (f; DAA; HH2)
- Sclerosis (f; DAA; JLH)
- Snake Bites (f; DAA; LMP)
- Sores (f; EB25:245)
- Sore Throat (f; KAB; RAI)
- Splenosis (f; BOU; DAW; UPW)
- Staphylococcus (1; RAI)
- Stings (f; DEM)
- Stomatosis (f1; RAI; VAD)
- Stones (f; AUS; DAA; JFM)
- Swelling (f; GMH)
- Throat (f; KAB)
- Uterosis (f; UPW)
- Vaginitis (f; VAD)
- Viruses (f1; RAI)
- Worms (f; LMP)
- Wounds (f; EB43:480)
- Yeast (f; NWO)
|
 To U.S. Users: To U.S. Users: This product
have not been evaluated by the U.S. Food & Drug Administration.
It is not intended to diagnose, treat, cure, or prevent any disease.
|
 Sourcing From
Sourcing From
The Peruvian
Amazon
 Although Avenca is native to
south Florida and Texas, as well as parts of Central and South America,
we source our variety of Avenca from the Peruvian Amazon.
 Recent Studies on Avenca
Recent Studies on Avenca
Sourced from PubMed
 Disclaimer: Disclaimer: The following citations provide findings on the
properties of Avenca and offer insights into prospective areas of future research.
These findings should not be inferred to provide the basis of medicinal claims,
nor should they be relied upon by the public, as such. Readers who want full access to
the PubMed database are encouraged to
register with NCBI.
 As of Jan. 2017, there were
94 citations on PubMed for Avenca. Below are list a few of the more notable:
-
Antiobesity and antihyperglycaemic effects of Adiantum capillus-veneris extracts: in vitro and in vivo evaluations. (2017)
[RESULTS: Like acarbose, A. capillus-veneris as well as chlorogenic acid, with respective IC50 values (mg/mL) of 0.8 ± 0.0 and 0.2 ± 0.0,
were identified as in vitro potent dual inhibitors of α-amylase/α-glucosidase. Unlike guar gum, A. capillus-veneris had no glucose diffusion
hindrance capacity. Equivalent to orlistat, A. capillus-veneris and its phytoconstituents inhibited PL in vitro with an ascending order of
PL- IC50 values (μg/mL): ferulic acid; 0.48 ± 0.06 < ellagic acid; 13.53 ± 1.83 < chlorogenic acid; 38.4 ± 2.8 < A. capillus-veneris;
1600 ± 100. Incomparable to acarbose or metformin and glipizide, A. capillus-veneris (125, 250 and 500 mg/kg b.wt) lacked antihyperglycaemic
efficacies in acute starch- or glucose-evoked postprandial hyperglycaemia increments in normoglycaemic overnight fasting rats.
Superior to atorvastatin; A. capillus-veneris exerted significant antiobesity (p < 0.001) with marked triacylglycerol-reducing capacities
(p < 0.001) in comparison to rats fed with HCD for 10 weeks. DISCUSSION AND CONCLUSION:
A. capillus-veneris, modulating pancreatic digestive enzymes, may be advocated as a combinatorial diabesity prevention/phytotherapy agent.]
-
The Effects of Adiantum capillus-veneris on Wound Healing: An Experimental In Vitro Evaluation. (2014)
[RESULTS: The aqueous part of A. capillus-veneris promoted significant angiogenesis (P < 0.05) through both capillary-like tubular formations
and proliferation of endothelial cells in vitro. In addition, in the tests for protection against damage to fibroblasts by oxygen free radicals,
aqueous and butanol fractions showed significant protective effects in the concentrations 50, and 500 μg/ml (P < 0.05) in comparison
with a control group. In the toxicity testing, it showed weak irritation in the Hen's egg test chorioallantoic membrane (CAM) bioassay at
the vascular level on the CAM of the chicken and no significant cytotoxicity in the MTT assays on normal human dermal fibroblasts.
CONCLUSIONS: Angiogenic effects and protective effects against oxygen free radicals suggested aqueous partition of A. capillus-veneris
local application for prevention of late-radiation-induced injuries after radiation therapy and healing of external wounds similar to bedsores and burns.]
-
Effect of Adiantum Capillus veneris Linn on an Animal Model of Testosterone-Induced Hair Loss. (2014)
[ABSTRACT: Androgenetic alopecia is the most common form of hair loss in men. The present study was designed to evaluate the hair growth-promoting
activity of a preparation of the Adiantum capillus-veneris Linn. (A. capillus-veneris) on albino mice using a testosterone-induced alopecia model.
Five groups of albino mice were studied: (A) Testosterone solution only (n=6); (B) Testosterone + Finasteride solution (2%) (n=6);
(C) Testosterone + vehicle (n=6); (D) Testosterone + A. capillus-veneris solution (1%) (n=6); (E) intact control (n=2, without testosterone).
Alopecia was induced in all intervention groups by testosterone 1.0 mg subcutaneous. A. capillus-veneris solution was applied topically to the back
skin of animals in the respective group. Hair growth was evaluated by visual observation and histological study of several skin sections via
various parameters as follicle density (number of follicles/mm) and anagen/telogen ratio. After 21 days, a patch of diffuse hair loss was seen in
animals received testosterone while animals treated with A. capillus-veneris showed less hair loss as compared to those treated with testosterone
only. The follicular density observed in the A. capillus-veneris-treated group was 1.92 ± 0.47, compared to 1.05 ± 0.21 in testosterone-group and
2.05 ± 0.49 in finasteride-treated animals. Anagen/telogen ratio was significantly affected by A. capillus-veneris, which was 0.92 ± 0.06 as compared
with 0.23 ± 0.03 and 1.12 ± 0.06 for testosterone and finasteride treated groups, respectively. According to visual observation and quantitative data
(follicular density and anagen/telogen ratio), A. capillus-veneris was found to possess good activity against testosterone-induced alopecia.]

-
In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris.
(2014) [ABSTRACT:
Adiantum capillus veneris is a medicinally essential plant used for the treatment of diverse infectious diseases. The study of phytochemical and
antimicrobial activities of the plant extracts against multidrug-resistant (MDR) bacteria and medically important fungi is of immense significance.
Extracts from the leaves, stems, and roots of Adiantum capillus veneris were extracted with water, methanol, ethanol, ethyl acetate, and hexane and
screened for their antimicrobial activity against ten MDR bacterial strains and five fungal strains isolated from clinical and water samples.
Ash, moisture, and extractive values were determined according to standard protocols. FTIR (Fourier transform infrared Spectroscopy) studies were
performed on different phytochemicals isolated from the extracts of Adiantum capillus Veneris. Phytochemical analysis showed the presence of
flavonoids, alkaloids, tannins, saponins, cardiac glycosides, terpenoids, steroids, and reducing sugars. Water, methanol, and ethanol extracts of
leaves, stems, and roots showed significant antibacterial and antifungal activities against most of the MDR bacterial and fungal strains. This study
concluded that extracts of Adiantum capillus veneris have valuable phytochemicals and significant activities against most of the MDR bacterial strains
and medically important fungal strains.]

 Extensive information about
Avenca is covered on the Raintree Forest website.
Even better, you can purchase Leslie Taylor's excellent reference book,
The Healing Power
of Rainforest Herbs: A Guide to Understanding and Using Herbal Medicinals at Amazon.
 See Wikipedia
article on this herb.
|


 Recent Studies on Avenca
Recent Studies on Avenca